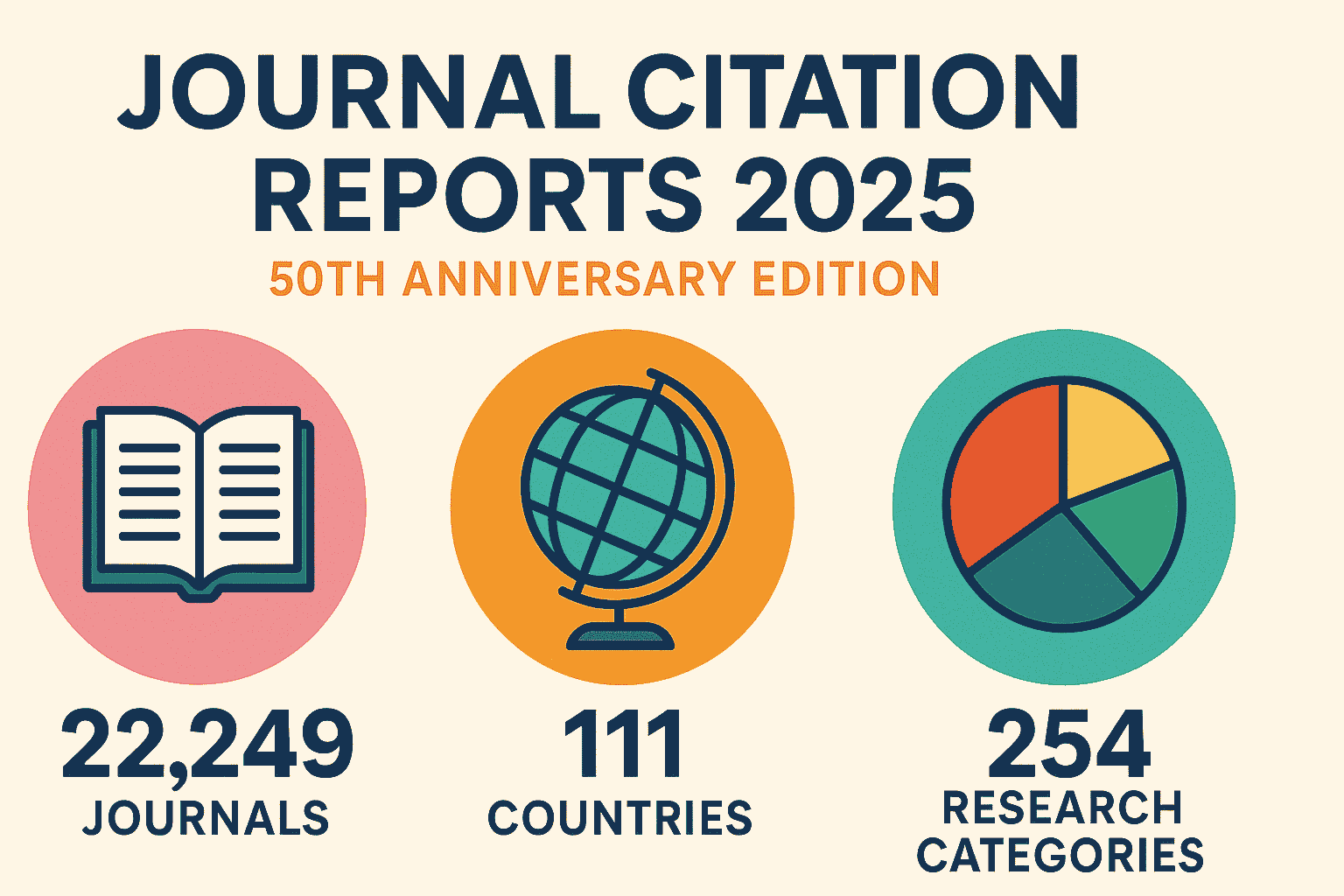
NIH restores grants to South Africa scientists, adds funding option for other halted foreign projects
A policy tweak to the ban on foreign “subawards” should allow clinical trials worldwide to continue
Summary
Recent adjustments to the US ban on foreign “subawards” are expected to ease restrictions on international clinical trials. While the initial ban threatened to disrupt global research collaborations, the revised policy clarifies permissible activities, ensuring that essential foreign-based clinical trial work can proceed. This clarification specifically addresses concerns that the broad interpretation of “subawards” would hinder the ability to conduct clinical trials in diverse populations and access critical research facilities abroad. The change aims to balance national security concerns with the need for collaborative, global research efforts to advance medical knowledge and improve healthcare outcomes.
Read more…
This post is part of “Science/Immunology News”, Follow for more…!!!